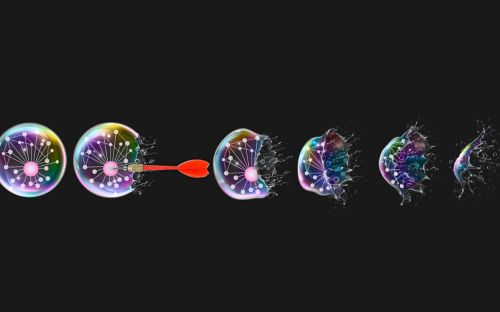
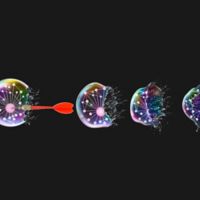
Researchers identify new molecular triggers for cell’s 'executioner'

Tudor Moldoveanu’s lab studies a form of cell death called necroptosis and how abnormal activation of some cell deaths can be linked with myriad disorders and diseases.
To protect the body, cells that are damaged or infected commit suicide through a process called necroptosis. However, this death program also plays a key role in a multitude of diseases. Cancer cells avoid destruction by shutting down necroptosis. On the other hand, abnormal activation of necroptosis has been linked to multiple sclerosis, Parkinson’s disease, ischemic tissue injury from blood flow loss and inflammation from infectious diseases. So, learning to understand and control necroptosis offers a highly promising route to treating a broad array of diseases.
Tudor Moldoveanu, Ph.D., of the Department of Structural Biology, and his colleagues are mapping the necroptosis control machinery. Their latest paper in the journal Cell Chemical Biology has identified a critical triggering mechanism for the process.
In their study, the researchers explored the role of molecules called inositol phosphates (IPs) in unleashing the cell-killing molecule, called MLKL, in necroptosis. MLKL’s lethal weapon is its “executioner domain” — the segment of the molecule that attacks and ruptures the cell membrane to kill the cell. Normally, this domain is kept under tight rein. Previous research by Moldoveanu and his colleagues revealed that it is somehow activated by IP molecules, but did not show which ones or exactly how they do it.
In the latest experiments, the researchers used genetic manipulation of cells and molecular structural analysis to zero in on how multiple IPs work together to unleash the executioner domain. In essence, researchers found that the different molecules constitute an “IP code” that works something like the combination to a safe. The IP molecules vary according to the attachment of various numbers of a chemical group called a phosphate to their structure.
“We now know that there are two events that activate MLKL,” Moldoveanu said. “One is the action of a kinase RIPK3. And the other is the binding of inositol phosphates.” Kinases are chemical switches that activate proteins. The finding of IP involvement was surprising, because scientists had believed simple molecules acted mainly in a support role in the cell, rather than being involved in signaling.
Moldoveanu plans to explore the “upstream” machinery of the IP code, to understand why it is so important in the necroptosis machinery. He theorizes that the IPs may constitute some kind of signal of how healthy a cell is; and their activation of necroptosis may reflect a damaged or infected cell that needs to be killed.
Essential ingredients, such as BBQ sauce, are a must for delicious ribs served at southern cookouts. Similarly, for a form of cell death known as necroptosis to proceed smoothly, inositol phosphate metabolites (IPMs) have been identified by McNamara et al. as essential ingredients in activation of the executioner of necroptosis, MLKL. The bottle label, showing the red pepper with a spark onto the subtle skull background, is metaphoric with MLKL activation by IPMs in necroptosis.
The researchers will also explore the executioner domain of MLKL to understand the structural details of how the IPs activate it and how it brings its membrane-popping weaponry to bear to kill the cell.
All these studies offer clues to understanding necroptosis, which could lead to drugs to control it, Moldoveanu said. The drugs would act to turn on or off the kinase switches responsible for producing the different forms of IP.
“Necroptosis clearly has potential in multiple therapeutic strategies for different diseases,” he said. “What particularly interests us here at St. Jude is how this knowledge might be used to treat cancers. Since necroptosis seems to work along with the immune response, it might combine with immunotherapy to kill tumors.”
On the other hand, he said, drugs to block IP production and shut down necroptosis could stop cell death in such disorders as multiple sclerosis, Parkinson’s disease and ischemic tissue injury from blood flow loss. Such drugs would aim to jam the kinase switches that produce the various forms of IP. The drugs might combine with those that inhibit the other MLKL activator, RIPK3, in a one-two therapeutic punch to block necroptosis.