Cancer-fighting ninja protein may offer clue to create self-harming cancer cells
Tudor Moldoveanu, PhD, right, discovered a ‘lone-wolf’ protein that can cause cancer cells to kill themselves.
If a cancer cell is the enemy we aim to destroy, the drugs we develop are the army to fight it. But cancer is sometimes elusive, and deceives us in our efforts to kill it.
Our body’s immune system, while typically very powerful, is useless against the cancer. An ally is needed, something that can deceive the deceiver.
It will have to be a covert operation by a single, highly effective assassin using an unsuspected mode of attack. It seems like such a long shot to find such help.
Now imagine we found it. That would be exciting, right?
My colleagues from the St. Jude Department of Structural Biology and I recently found a protein that could act alone and possibly trigger apoptosis, a process of programmed cell death.
Understanding apoptosis is key — when the body needs to rid itself of cells it no longer needs or that are not functioning normally and could potentially cause harm, it uses a built-in mechanism that causes the malfunctioning or unwanted cells to die.
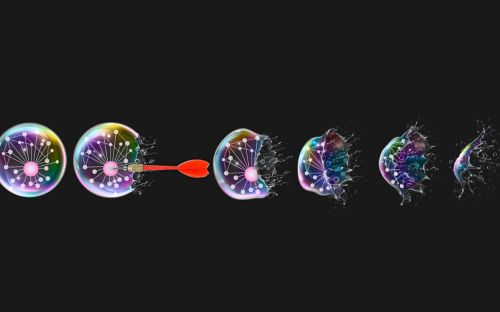
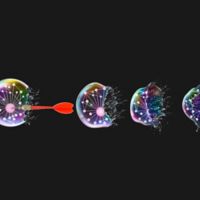
The most common way for this to happen in mammalian diseases like cancer is by mitochondrial apoptosis, where the membrane of the mitochondria (the power source of the cell) is pierced by certain proteins like the one central to this study - BOK or BCL-2 ovarian killer - releasing key proteins through the holes in the mitochondria that initiate destruction of the cells.
Cancer cells, unfortunately, defend themselves by switching off apoptosis. Many existing cancer drugs try to turn that switch back on. BOK is different.
It does its own thing. It’s not controlled by the apoptosis machinery — it is held in check by proteasome, a protein-shredding mechanism inside the cell. When signaled for apoptosis, BOK levels may increase overcoming the ability of the proteasome to properly destroy it.
Using nuclear magnetic resonance spectroscopy, we detailed the structure of BOK proteins for the first time and better understand how it works. Based on intrinsic features of its structure we discovered that BOK can spontaneously pierce mitochondrial membrane to turn on cell death.
This may lead to a new pathway to treat cancer. Inhibiting proteasome in cancer cells is one step. Instead of activating apoptosis via the well characterized cellular switches, new treatments could work by having the drugs plug into the BOK protein itself and stabilize it against proteasome and allow BOK levels to rise and initiate apoptosis.