Length matters in dipeptide repeat polypeptides – and can be a way to treat ALS

Researchers from Richard Kriwacki’s (left) lab found out the molecular mechanisms involved in mutations that may lead to Lou Gehrig’s disease.
The door has opened for possible new approaches to the diagnosis and treatment of amyotrophic lateral sclerosis (ALS), often called Lou Gehrig’s disease.
A new 1.1 GHz nuclear magnetic resonance spectroscopy system, which will be installed at St. Jude, helped solidify the findings which were published in the journal Molecular Cell.
Arginine, DPR length and ALS
Mutations in the gene C9orf72 are associated with about 35 percent of ALS cases, making it the most common genetic cause of ALS and a related neurological disease, frontotemporal dementia. This mutation causes a sharp increase in short, repetitive DNA sequences and the subsequent formation of abnormal, repetitive proteins called dipeptide repeat polypeptides.
Some DPRs, particularly those enriched in the amino acid arginine, are exquisitely toxic. Until this work, we didn’t know the details of the molecular mechanisms involved.
Here’s what happens when Corf72 mutates
We showed that the toxic DPRs undermine cell survival by disrupting the function of a key protein, nucleophosmin. This protein helps the nucleolus maintain its shape and function even though it lacks a membrane. The nucleolus is where cellular protein factories—called ribosomes—assemble.
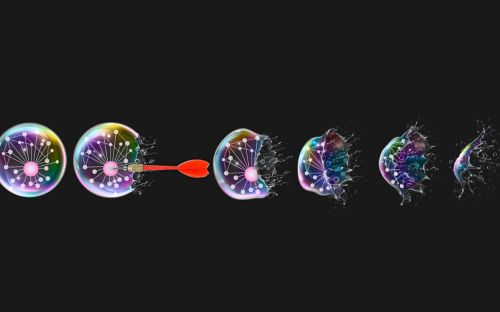
Membrane-less organelles like the nucleolus rely on the same process that explains why oil and water separate. The process, known as liquid-liquid phase separation, allows cells to temporarily sequester or concentrate components to respond rapidly to changing conditions.
A segment within the C9orf72 gene is commonly repeated up to ~30 times. Those who develop ALS or FTD, though, can have hundreds, maybe thousands of the repeats, which underlies DPR formation. DPRs disrupt nucleolar function by interacting with key regions of nucleophosmin. These interactions displace nucleophosmin binding partners that help maintain nucleolar structure and ribosome assembly.
DPRs disrupt that process, causing regions of the nucleolus to dissolve. The greater the DPR length and concentration, the faster parts of the nucleolus dissolve.
We also discovered that toxic DPRs disrupt cell function by binding and sequestering ribosomal RNA, which is essential for ribosome synthesis.
During this project, the NMR instrument destined for St. Jude was still at the manufacturer in Zürich, Switzerland. We shipped our samples to Zürich so that we could reveal in greater detail how toxic DPRs interact with particular regions of nucleophosmin.
The research provides new directions for thinking about diagnosis and treatment of these devastating diseases. For example, since we show DPR length is a prognostic of toxicity, future diagnostic tests may measure DPR length to screen for ALS in families with a history of the disease or in those determined to be genetically at risk.